The Forward Reaction Is Best Described as
Up to 24 cash back C endothermic reaction in which energy is released D endothermic reaction in which energy is absorbed 13The forward reaction is best described as an A A B B C C D D 14Which arrow represents the activation energy for the forward reaction. O Equilibrium is reached when the reaction stops.

Sbi Feedback Ppt Slides Business Powerpoint Templates Presentation Powerpoint Templates
Backward reaction is when reaction goes from products to reactants.

. A potential energy of the reactants only. Forward reaction is the reaction starting from the initial reactants to products. The best statement which describes a reaction in a state of equilibrium is letter D.
The forward reaction is exothermic and the reverse reaction can be either. Exothermic reaction in which energy is absorbed D. A property of a system that depends on the path taken between the initial and final states.
The rate of the forward reaction equals the rate of the reverse reaction and the concentrations of products and reactants are constant. D The forward reaction is endothermic and the reverse reaction can be either endothermic or exothermic. C The forward reaction is exothermic and the reverse reaction can be either exothermic or endothermic.
When a forward reaction event from B i to B i 1 is selected whose rate is denoted as r i one CTB bound to i number receptors is randomly selected and another free receptor will be bound to it. A A B B C C D D 15The potential energy diagram of a chemical reaction is shown below. 3 The Forward Reaction Is Best Described As An 1 Exothermic Free PDF eBooks.
8 HIV is the virus that causes AIDS. A endergonic G 0 B endergonic G 0 C exergonic G 0 D exergonic G 0. A system at equilibrium is in a state of dynamic balance with forward and reverse reactions taking place at equal rates.
The sum of kinetic and potential energy contained in a. A B C D heat Which statement best describes this reaction. The forward reaction.
Endothermic reaction in which energy is absorbed 38. The reaction has stopped because all reactants have been used up. 4 The forward reaction is endothermic and the reverse Δ ΔS Δ Δ.
At STP a sample of which element has the highest enfropy. 2 The forward reaction is exothermic and the reverse reaction is always endothermic. The eaction is best described as 1.
7 Which of the following terms best describes the forward reaction in the figure. Mar 15 2022. Exothermic reaction in which energy is released C.
4 The additional bromine ions. 3 by adding water h2O. Activation Energy kJ ΔH kJ A.
Which of the following describes the forward catalyzed reaction. The forward reaction is exothermic ΔH 92kJmol so the reverse reaction is endothermic ΔH 92kJmol. Chemistry questions and answers.
3 The forward reaction is exothermic and the reverse reaction can be either exothermic or endothermic. O There is only one set of equilibrium concentrations that equals the Kc value. At equilibrium the rate of the forward reaction equals the rate of the reverse reaction.
Up to 24 cash back 18. 2 More liquid water molecules will change to water vapor until a new equilibrium is reached. Here it is required to check whether there are any free receptors sufficiently close to the selected binding spot which is determined by the distance between the binding spot to free.
Which of the following terms best describes the forward reaction in Figure 81. If an equilibrium system is subjected to a change in conditions that affects these reaction rates differently a stress then the rates are no longer equal and the system is not at equilibriumThe system will subsequently experience a net reaction in the. Given the balanced equation representing a reaction.
O At equilibrium the rate of the forward reaction is the same as the rate of the reverse reaction. The forward reaction is best described as an A. Given a reversible reversible reaction you may arbitrarily choose which chemicals to consider the reactantsproducts since the reaction goes from both the reactants to the products and from the products to the reactants.
The appearance Will continue to change as the forward reaction continues. The rate of the forward reaction is slower than the rate of the reverse reaction. A separator cools the reaction mixture which liquifies the ammonia enabling it to be removed from the non-condensed.
The forward reaction is exothermic and the reverse reaction is always endothermic. The statement At equilibrium the rate of the forward reaction is the same as the rate of the reverse reaction best describes general equilibrium. Up to 24 cash back Base your answer on the potential energy diagram of a chemical reaction.
Which statement best describes general equilibrium. Equilibrium reversible and the forward and reverse reactions occur simultaneously at the same rate. Two substances are dissolved in water and start to react which statement accurately describes the appearance of the solution when it reaches equilibrium a.
Which statement best describes this reaction. O At equilibrium the total concentration of. Chemical reactions can be classified into one of two broad categories.
The forward reaction is exothermic and the reverse reaction is always exothermic. A property of a system that depends only on the current state of the system not on the path the system took to reach that state. A substance that increases the rate of a reaction without appearing in the equation for the overall reaction is aan A.
Answer 1 of 6. 1 The forward reaction is exothermic and the reverse reaction is always exothermic. E chemical equilibrium AG 0 C D Free Energy Progress of the Reaction - D.
Simply put the forward reaction starts with what you consider to be the reactants. 1 The rate of the forward reaction equals the rate of the reverse reaction. Posted on April 07 2016.
If the equilibrium of a reaction is said to lie to the right that reaction could be described as reactant- favored. This phrase comes from Le Châteliers Principle. In the mid-1990s researchers discovered an enzyme in HIV called protease.
Endothermic reaction in which energy is released B. A endergonic AG B exergonic AGO AB C endergonic AG 0 D exergonic AG d. As a reaction proceeds in the forward direction to establish equilibrium the value of Q - increases If a system at equilibrium contains gaseous reactants or products a decrease in the volume of the system will cause the system to shift in the direction the produces - moles of gas whereas an increase in volume causes a shift in the direction that produces - moles of gas.
At equilibrium the concentration of the reactants must equal the concentration of the products. A chemical process in which the rate of forward reaction is equal to the rate of backward reaction.

Chemical Equilibrium Notes And Ice Method Chemistry Notes Engineering Notes Chemistry Lessons

Skincare Planner Beauty Routine Planner Printable Skin Care Planner Glow Up Planner Beauty Journal Makeup Tracker Skincare Journal Beauty Routine Planner Beauty Journal Skin Care

Bohr Atomic Models Worksheet Answers Atom Physics Worksheet Chemistry Notes Teaching Chemistry Chemistry Education

Pin On Reactions Of Carboxylic Acids And Their Derivatives Practice Problems

Welcome To Learnapchemistry Com Ap Chemistry Question Paper Ap Chem

Gabriel Synthesis Of Amines Teaching Chemistry Chemistry Lessons Organic Chemistry

Cgp Igcse 9 1 Edexcel International Gcse Chemistry Biology Physics 3 Books Collection Set Grade 8 9 Targeted Exam Practice Workbookdefault Title Gcse Chemistry Exam Chemistry

Transesterification Reaction Organic Chemistry Medicine Studies Chemistry

My Best Life Journal Living Your Best Life Journal Ships Out Jan Set In Soul Life Journal Love And Forgiveness Live For Yourself

Kenneth Cole Reaction Speed Ball Lo Sneaker Kenneth Cole Reaction Shoes Kenneth Cole Reaction Sneakers Fashion
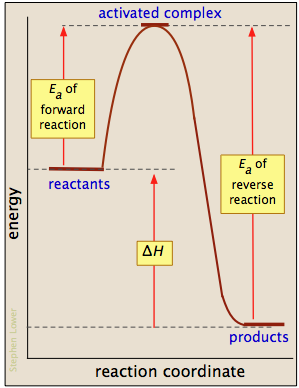
If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Compare With The Activation Energy For The Reverse Reaction B A Socratic

Reversible Reaction Easy Science Biology Notes Ap Chemistry

Pin By Kathleen Zedalis On Science A Nuclear Reaction Extreme Behavior

Difference Between Reversible Reaction And Irreversible Reaction Chemistry Chemical Equilibrium Chemical Chemistry Chemical Reactions

Law Of Mass Action Chemical Equilibrium Chemistry Animation Chemistry Lessons Chemistry Equilibrium

Chemistry Notes Chemistry Pdf Chemical Equilibrium And Ice Method Chemistry Notes Chemistry Chemistry Lecture

Effect Of The Change In Concentration On Equilibrium State Equilibrium Le Chatelier S Principle Concentration

Study Notes Study Notes Study Studying Inspo

Chemical Reactions Types Worksheet Chemical Reactions Types Worksheet Awesome Types Chemical Chemistry Lessons Teaching Chemistry Science Chemistry
Comments
Post a Comment